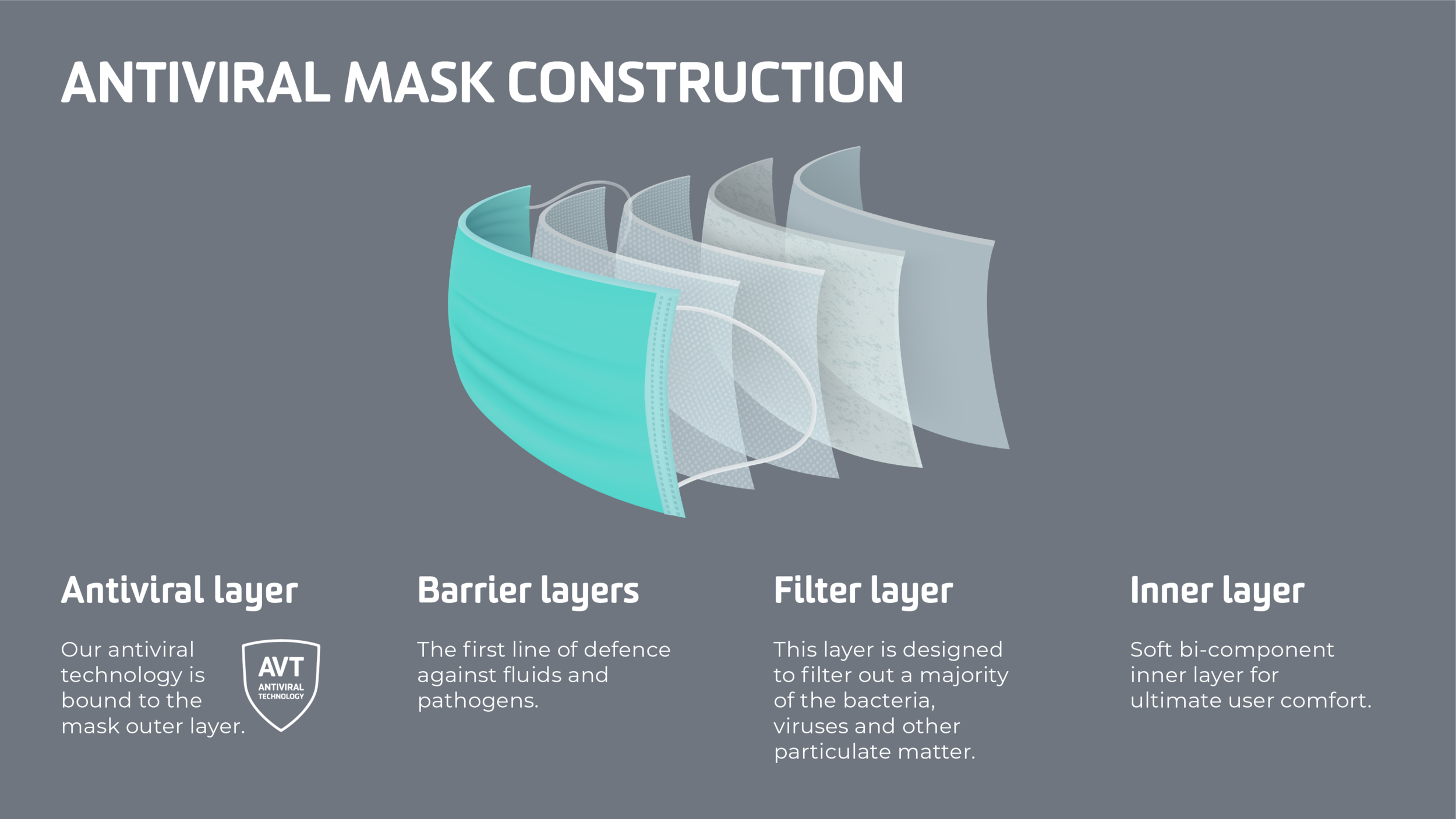
ANTIVIRAL TYPE IIR FACEMASK
KILLS 99.99% OF SARS-COV-2 (COVID-19) AT JUST 10 MINUTES
proven to stop cross contamination
Sanitas has developed an ANTIVIRAL Facemask
RAPID KILL innovation
Kills 99.99% of SARS-CoV-2 (COVID-19) at just
10 minutes+ 8 hours Antiviral activity
Patented UK invention
Uses “activated oxygen” driven by daylight
Unique NON-LEACHING technology
The antiviral agent is bound to the mask
No exposure to wearer or environment
Fully compliant Medical Device registered in the UK and EU
SCIENTIFIC MASK STUDIES
Previous work by university scientists highlight ways masks can be better:
Respiratory viruses (such as Influenza and coronaviruses) have been shown to collect on the outside of facemasks during wear
Coronavirus SARS-CoV-2 has been proven to survive on standard facemask for upto 7 days
Transfer (“cross contamination”) from masks has been demonstrated when they are removed
Onto clothes and skin of the wearer
ANTIVIRAL TESTING
Independently validated. Positive impact demonstrated
Independent bespoke testing shows 99.99% kill of SARS-CoV-2 at 10 min
Have further in house and fully independent testing to ISO 18184 (to share)
Sanitas’ in house expertise allows further high impact experiments
Antiviral action proven for at least 8 hours (vs 1 hour standard test)
Kill of viral aerosols shown (vs standard large drops)
Crucially ZERO VIRAL TRANSFER from Protect AV mask when touched
(100,000’s of infectious viruses moved from a standard mask)
Commercial volumes and retail packs available
PATENTS
Patents secured protecting our technology in a range of applications
Antimicrobial Gloves
Antimicrobial Facemask
Antimicrobial Gown
Antimicrobial packing materials
Regulatory
Protect Type IIR Antiviral mask is a CE Marked Medical Device
MHRA Registered
Registered in the EU
Compliance with all required International Standards
ISO10993 Biocompatibility
EN14683 Blood Penetration, Filtration
ISO14971 Medical Device Risk Management
SUMMARY OF ANTIVIRAL TYPE IIR FACEMASK
RAPID KILL innovation
Kills 99.99% of SARS-CoV-2 (COVID-19) at just
10 minutesPatented UK invention
Unique NON-LEACHING technology
Fully compliant Medical Device registered in the UK and EU
Contact us for orders or enquiries